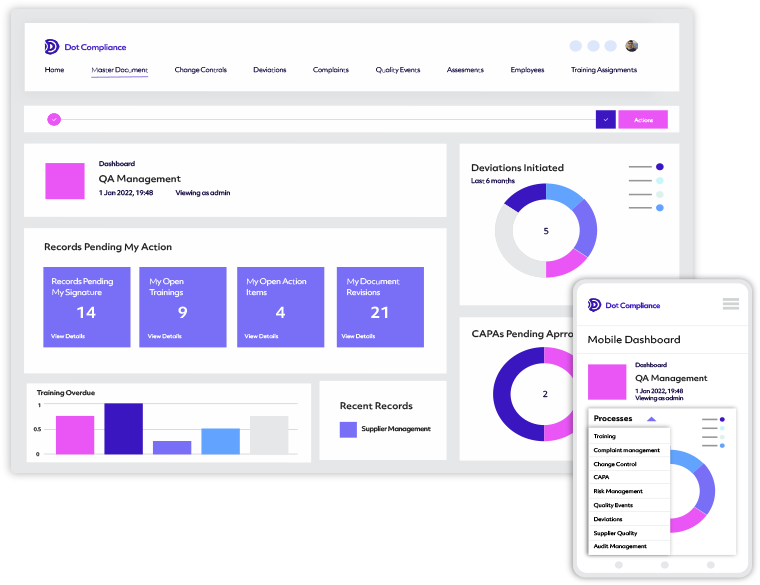
The Ready to Use,
AI Powered eQMS
Streamline quality and compliance processes, and uncover hidden insights with the leading eQMS for the life science industry.












Distinctly Different
eQMS deployment doesn’t have to be lengthy, complex and expensive.
Ready to Use eQMS
Pre-configured based on best practice processes’ powered by the Salesforce.com platform.
Seamless Deployment
Install quickly and get up and running within a week with minimal set up required.
Most Cost Effective
Eliminate expensive and time consuming customization and solution delivery costs.
Find the Dot Compliance Solution For You
Addressing any eQMS need, for any size company with unmatched breadth and depth process coverage
QMS Xpress
Set your eQMS foundation with a comprehensive set of ready to use core quality processes. Learn More
Compliance Xpand
Build upon core eQMS, expanding into other manufacturing quality and compliance specialty areas. Learn More

Enterprise Xact
Tailored eQMS, with customized and configured quality and compliance processes based on your unique requirements. Learn More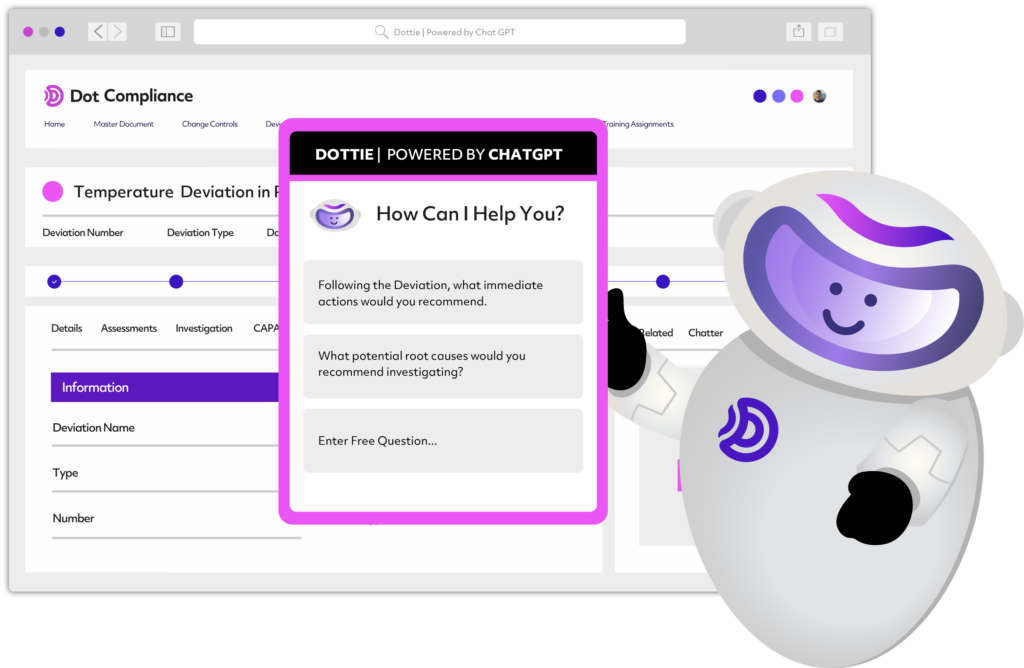
Harness the power of Ready to Use AI
The first ever eQMS platform with generative and predictive AI capabilities.
Core eQMS out of the box ready for deployment
Extensive set of off-the-shelf ready eQMS and compliance processes that include full product validation packages.

Dot Compliance has helped us be more efficient in our processes, while also maintaining a high level of compliance in a highly regulated industry.